MGB Eclipse® Probes and companion primers
MGB Eclipse probes and companion primers are manufactured under ISO 13485 conditions to meet your specific qPCR needs. Build your custom genotyping assays more easily with our variety of fluorophore and yield options.
Ordering
MGB Eclipse is a registered trademark of ELITech Group.
MGB Eclipse Probes
Manufactured under ISO 13485 conditions.
| Normalized Final Yield (nmol) | ||||
|---|---|---|---|---|
| Dye | Quencher | 6 | 20 | 50 |
| FAM | MGB Eclipse | - | - | - |
| HEX | MGB Eclipse | - | - | - |
| TET | MGB Eclipse | - | - | - |
| YAK | MGB Eclipse | - | - | - |
GMP companion primers
Product details
We have combined our proven oligo manufacturing expertise and ISO 13485 production processes to deliver MGB Eclipse Probes and companion primers. Together, these probe and primer pairs are well-suited for genotyping qPCR research applications. Our range of fluorophore options [FAM, HEX, TET, and Yakima Yellow® (EliTech Group)] help to ensure compatibility with your instrument and more easily design multiplex assays.
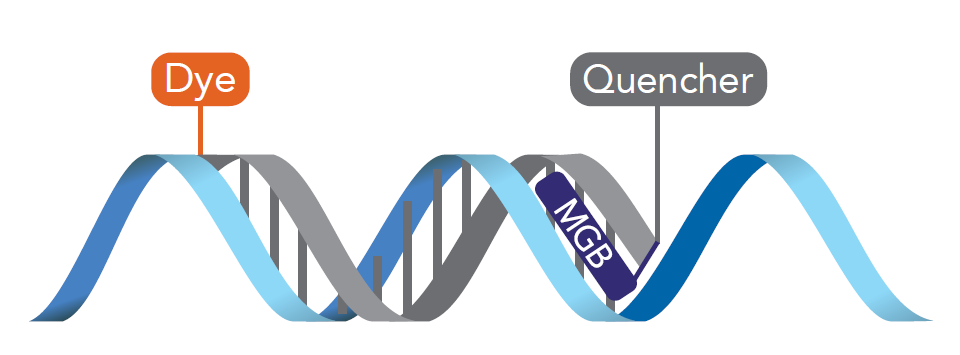
Figure 1. MGB Eclipse Probes. The incorporation of a minor groove binder (MGB) stabilizes probe-target hybridization and increases melting temperature, allowing the use of shorter probes which are better suited for allelic discrimination and targeting AT-rich regions in qPCR assays [1].
Freedom™ Dyes—Free of patent restrictions
Among our many available modifications, Freedom Dyes are fluorophores that have no patent licensing restrictions from IDT or any third-party company. Many common dyes are available in the portfolio, including FAM, HEX, JOE™ (Thermo Fisher Scientific™), MAX, TET, ROX, and TAMRA. Freedom ATTO™ (ATTO-TEC GmbH) dyes can be used as alternatives for VIC® (Thermo Fisher Scientific), LIZ® (Thermo Fisher Scientific), and Alexa Fluor® (Thermo Fisher Scientific) dyes. To learn more about Freedom Dyes, click here.
GMP refers to products manufactured under ISO 13485:2016 QMS. Purchaser is solely responsible for all decisions regarding the use of these products and any associated regulatory or legal obligations for their legal marketing.
Product data
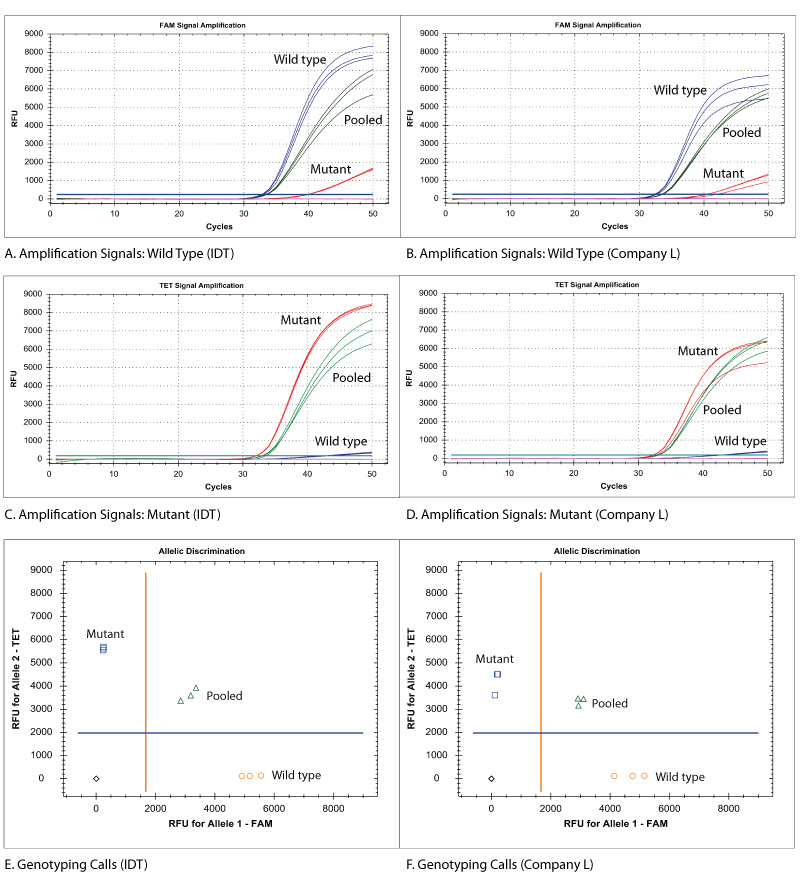
Research assays using MGB Eclipse Probes and companion primers from IDT produced identical results to industry standard assays when making genotyping calls for KRAS variants (Figure 2E-F). End-point fluorescent signal intensities were similar or higher using MGB Eclipse Probes compared to the third-party assay results (Figure 2A-D).
Figure 2. Comparison of signal amplification and genotyping results using IDT MGB Eclipse Probes or third-party products. KRAS G12R assays comprised of MGB Eclipse Probes (FAM dye—wild-type probe; TET dye—mutant probe) and primers manufactured by IDT or third-party assays for Research Use Only were used. (A-F) Reactions (10 μL) were run in triplicate with 104 copies of wild-type, mutant, or pooled wild-type/mutant template (gBlocks™ Gene Fragments; IDT) and TaqMan® Gene Expression Master Mix (Thermo Fisher Scientific) on a CFX384Touch™ Real-Time PCR Detection System (Bio-Rad). Cycling conditions were 3 min. 95°C; 50 x (10 sec. 95°C, 30 sec. 60°C). (A-D) Amplification curves for wild-type and mutant alleles demonstrated comparable or better results when using MGB Eclipse Probes from IDT (A: wild-type; C: mutant) versus third-party assays for Research Use Only (B: wild-type; D: mutant); n = 3. (E-F) Clear genotyping calls were made when using MGB Eclipse Probes from IDT (E) and third-party assays for Research Use Only (F); n = 3.
Services
Manufacturing process and traceability
When you order oligos manufactured under IDTs ISO 13485 QMS, you have the opportunity to monitor your oligos every step of the way. The process is governed by the Device Master Record Index (DMRI)—our collaborative playbook for how we produce your oligos every time you order.
We provide resources to help you define and monitor many aspects of your product's manufacture. As a GMP customer of IDT, you will have access to:
- LIMS batch records for every order
- Hard-copy analytical QC documentation on every oligo
- Long-term quality agreements and contracts for access to a stable supply of high-quality GMP oligos
Legal and regulatory guidance
Our facilities are ISO 13485:2016 certified. We maintain an "open door" audit policy.
- On-site product or process audits with our Quality Assurance team
- Reliable product-release process managed by our Order Verification and Release team
Request a consultation
Our team of experts is happy to meet with you to discuss any questions or special requests you may have. Reach them anytime using the IDT Help Request Form.
Resources
Related products
References
- Kutyavin IV, Afonina IA, Mills A, et al. 3’-minor groove binder-DNA probes increase specificity at PCR extension temperatures. Nucleic Acids Res. 2000;28(2):655-61.
MGB Eclipse is a registered trademark of ELITech Group.
GMP refers to products manufactured under ISO 13485: 2016 QMS. Purchaser is solely responsible for all decisions regarding the use of these products and any associated regulatory or legal obligations for their legal marketing.